2024-07-22
AlfaDAX online system (https://store.greatbay-bio.com/) offers global users efficient and convenient biopharmaceutical molecule druggability assessment services, helping customers mitigate risks through molecular modification and expedite new drug development.
The AlfaDAX online system predicted the aggregation and precipitation of 28 innovative molecules, which were subsequently verified through wet lab experiments. The prediction accuracy for molecules prone to aggregation and precipitation was 100%, while for those not prone to aggregation and precipitation, the accuracy was 78.6%, resulting in an overall accuracy of 89.3%. Comparative analysis with data from six public antibodies validated the AlfaDAX system's capability to assess druggability and preemptively identify drug risks, thereby reducing costs and enhancing efficiency.
Prediction of aggregation and precipitation of 28 innovative molecules
As shown below, the AlfaDAX online system flagged 14 innovative molecules with a high aggregation score, indicated by a red warning. These molecules were later assessed for turbidity and subjected to SEC analysis in wet lab experiments, confirming that all exhibited aggregation and precipitation. The AlfaDAX online system demonstrated a 100% prediction accuracy for molecules with aggregation and precipitation, allowing early risk detection and significantly reducing the burden of wet lab experiments.
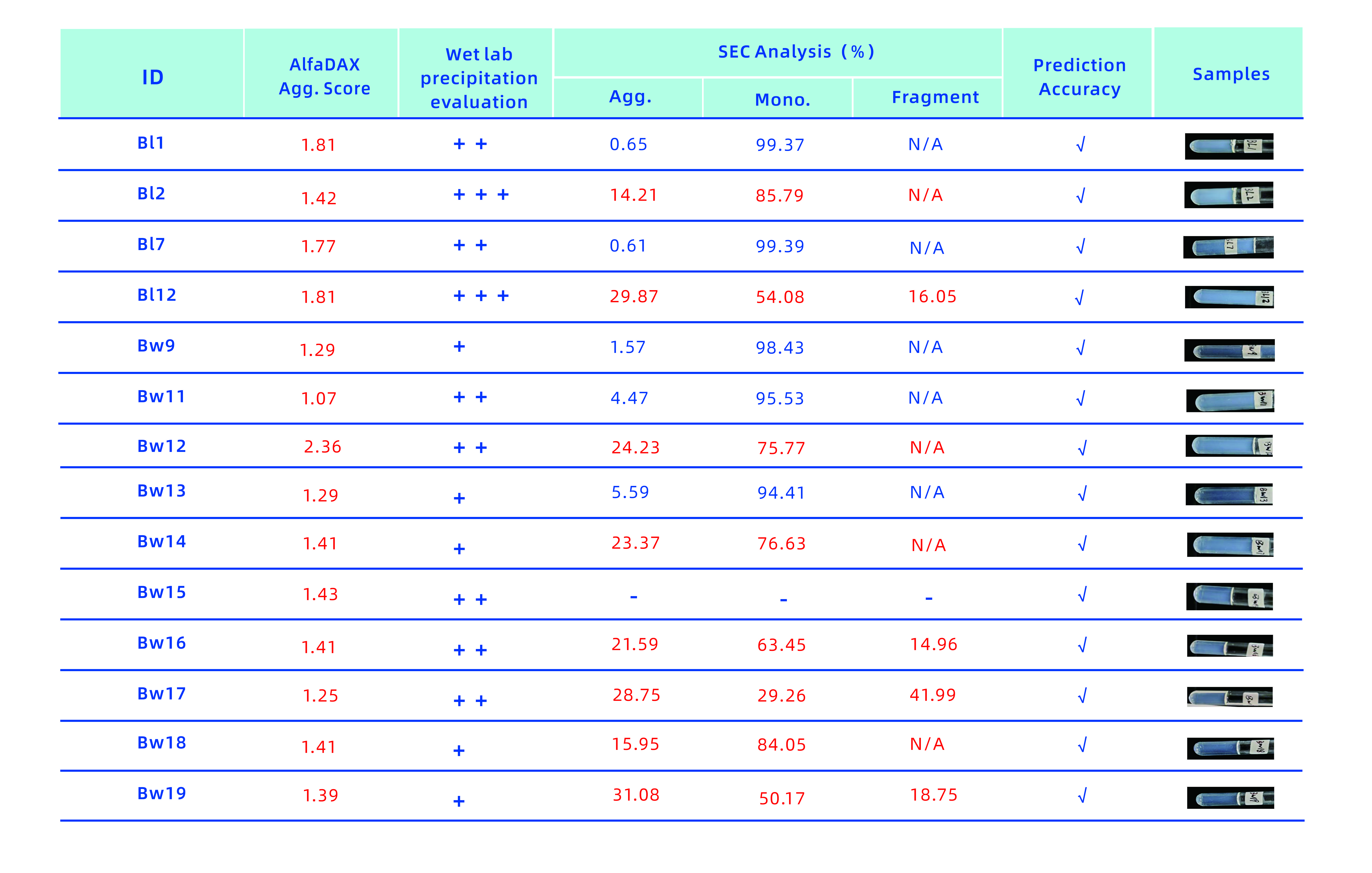
(The accuracy rate of prediction of aggregation and precipitation of the first 14 innovative molecules reached 100%)
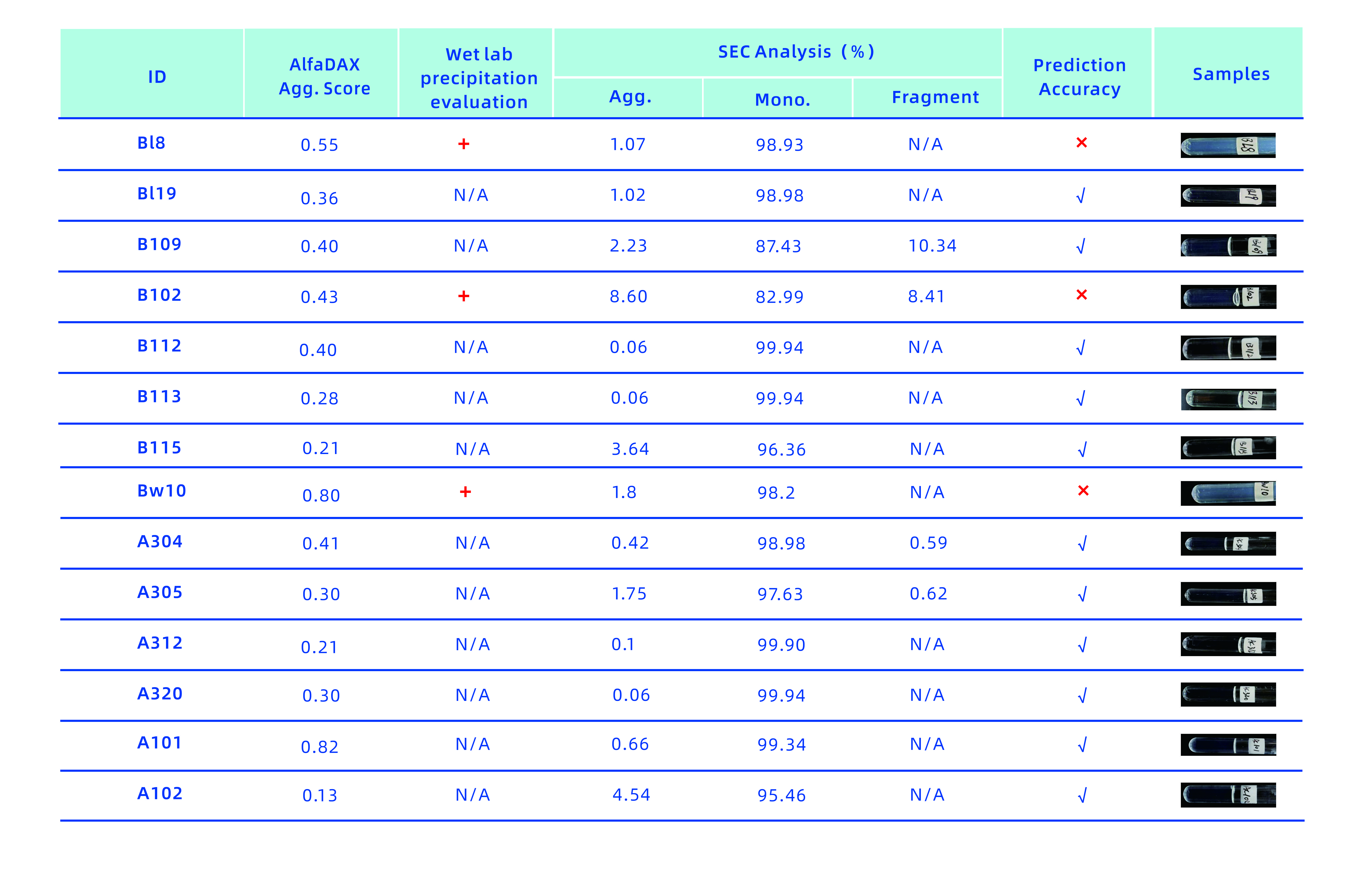
(The accuracy rate of prediction of aggregation and precipitation of another14 innovative molecules reached 78.6%)
Assessment results of 6 public drug molecules
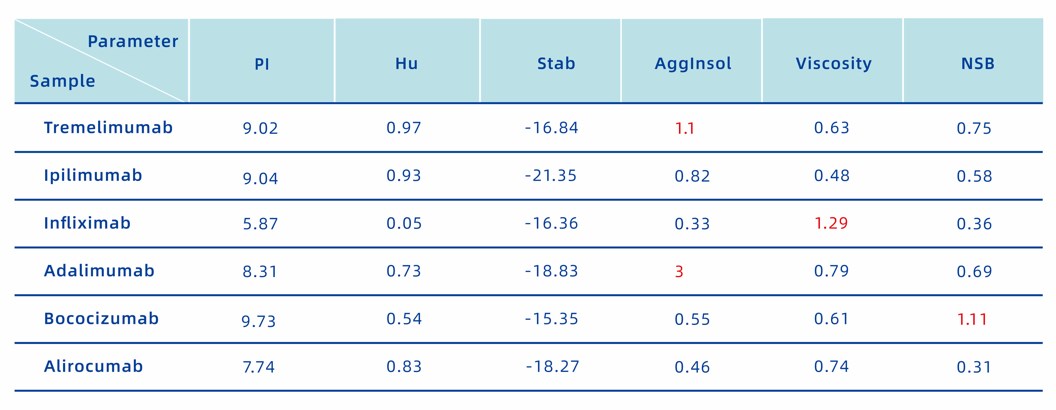
Tremelimumab and Ipilimumab are both marketed antibodies against CTLA4. AlfaDAX predicts that Tremelimumab is prone to aggregation, while Ipilimumab is not, which is consistent with the actual situation [1]. Accordingly, Ipilimumab is more widely used than Tremelimumab. On March 25, 2011, the US FDA approved Ipilimumab (trade name Yervoy) for the treatment of advanced melanoma. The administration method is intravenous injection. It is suitable for patients with unresectable or metastatic melanoma, intermediate-risk or low-risk advanced renal cell carcinoma, and certain types of metastatic colorectal cancer. Tremelimumab is mostly used in metastatic melanoma research and needs to be used in combination with Durvalumab to treat solid cancers such as liver cancer and lung cancer.
Infliximab and Adalimumab are both marketed antibodies against TNFA, but Infliximab has a higher viscosity [2]. The maximum recommended concentration of infliximab is 4mg/ml [3], which greatly limits its efficacy, while the maximum recommended concentration of Adalimumab can reach 100mg/ml [4]. This is one of the main reasons why Adalimumab can continue to be the best-selling antibody drug of the year, while Infliximab does not. Although fully humanized Adalimumab can be widely used, like mouse-derived Infliximab, it still has serious immunogenicity problems. About 28% of patients will develop drug-resistant antibodies within three years after injection of Adalimumab, which affects efficacy and causes safety problems [5]. The reason is just as predicted by the AlfaDAX online system. Adalimumab is easy to attract each other through electrostatic force and aggregate. Under pressure such as oxidation and stirring, it is extremely easy to produce small aggregated particles of varying degrees. These small aggregated particles of varying degrees are the main reason for causing different degrees of immunogenicity [5].
Bococizumab and Alirocumab are both PSCK9 antibodies. Bococizumab was halted in Phase III clinical trials, while Alirocumab was successfully launched on the market. Through AlfaDAX online system analysis, we found that Bococilizumab has a strong nonspecific binding tendency, which affects efficacy and produces toxic side effects [6].
Summary
AlfaDAX system predicts the aggregation and precipitation of 28 innovative molecules with an accuracy rate of 89.5%, aiding customers in early risk mitigation through molecular modification and accelerating the new drug development process. Please feel free to try the online system and provide feedback to us.

References
1.step, P., Caffry, I., Yu, Y., Sun, T., Cao, Y., Lynaugh, H., ... & Xu, Y. (2015, May). An alternative assay to hydrophobic interaction chromatography for high-throughput characterization of monoclonal antibodies. In MAbs (Vol. 7, No. 3, pp. 553-561). Taylor & Francis.
2.Thorsteinson, N., Gunn, J. R., Kelly, K., Long, W., & Labute, P. (2021, January). Structure-based charge calculations for predicting isoelectric point, viscosity, clearance, and profiling antibody therapeutics. In MAbs (Vol. 13, No. 1, p. 1981805). Taylor & Francis.
3.https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/103772s5401lbl.pdf
4.https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/125057s423lbl.pdf
5.Heljo, P., Ahmadi, M., Schack, M. M. H., Cunningham, R., Manin, A., Nielsen, P. F., ... & Jiskoot, W. (2023). Impact of stress on the immunogenic potential of adalimumab. Journal of Pharmaceutical Sciences, 112(4), 1000-1010.
6.Makowski, E. K., Chen, H., Lambert, M., Bennett, E. M., Eschmann, N. S., Zhang, Y., ... & Tessier, P. M. (2022, December). Reduction of therapeutic antibody self-association using yeast-display selections and machine learning. In MAbs (Vol. 14, No. 1, p. 2146629). Taylor & Francis.

Kingsley Leung, Chairman of Great Bay Bio, Awarded the FHKI X OCBC Hong Kong Innopreneur Awards 2025, Recognizing Outstanding Innovation
2025-11-14

Great Bay Bio and Taron Form Strategic Partnership to Advance HK Biopharmaceutical Innovation
2025-11-11

Great Bay Bio and Eureka Therapeutics Partner to Develop AI-Powered Antibody Discovery Model
2025-06-20